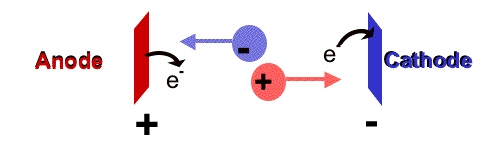
DEMos: Charged Up on ElectrophoresisChE 1101 Fall 2005Electrophoresis is the migration of charged particles or molecules through a solution under the influence of an applied electric field usually provided by immersed electrodes. Particles with a positive charge go to the cathode (negatively charged electrode) and negative charges go to the anode (positively charged electrode). Electrophoresis is widely known in its role in determining the human genome. This method can separate proteins or nucleic acid chains; by analyzing the rate of movement of each component in a gel, the molecular structure can be deduced.
The electrophoretic mobility, μ, of ions in solution can be obtained from experimental data by taking the velocity, v, divided by the electric field, E.
The electric field is the voltage divided by the length between the anode and the cathode.
Group A 10
mL NaCl 0
mL E-pure H2O
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results | |
| Step 1. | The voltage that will be applied is __________________ Volts |
| Step 2. | The total length of the tray is ____________________cm The electric field is _________________Volts/cm |
| Step 3. | Amount of Bromothymol blue indicator _______________ mL |
| Step 4. | Amount of NaCl solution ________________ mL |
| Step 5. | Total liquid in the electrophoresis tray_________________ mL |
Hash mark (cm) |
Time (min: sec) |
pH (optional) |
1 cm |
|
|
2 cm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We want to find out how fast the ions move in the electric field. This
can be done by plotting the distance versus time in Excel in
the following way.
Import the data into Excel and use the trendline function to draw a best fit line through your points. The rate of distance change or velocity can be found from the slope of the line.
Velocity, v, =
___________________________________![]()
Now from the velocity, we can calculate the mobility of the ions (page 1).
Mobility, μ,
= ___________________________________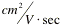
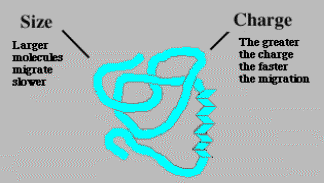
Mobility is important because we can compare this value to other data to tell
if our charged molecule is larger or smaller or if it has more of a charge. A
fragment of DNA (DeoxyriboNucleic Acid) has a mobility of .008 ![]() . What
does this tell us about its size or charge compared to our NaCl ions?
. What
does this tell us about its size or charge compared to our NaCl ions?
____________________________________________________
Your team has been assigned to one of 4 groups each with a different amount of NaCl. The percent of NaCl in the total fluid is shown in the table below. Calculate your mobility using the equations and your data. Report this value (with units) on webCT. After Friday, October 7th, look at the compiled results from other groups on webCT and write it in table form as is shown below.
|
Group A |
Group B |
Group C |
Group D |
Bblue (mL) |
10 mL |
10 mL |
10 mL |
10 mL |
NaCl (mL) |
10 mL |
7 mL |
5 mL |
3 mL |
E-pure H2O |
0 mL |
3 mL |
5 mL |
7 mL |
Weight % NaCl |
1.46 % |
1.02 % |
0.73 % |
0.44 % |
Mobility, m |
|
|
|
|
What do you notice about how the mobility changes with percent of NaCl? ____________________________________________________
Please clean up your experimental area being careful to avoid spills. Pour the used chemical into the designated waste containers. Chemicals need to be disposed of in an environmentally friendly way.
To deal with the pH data, refer to the extra handout that goes over how pH log scale works and what the acid / base pH numbers mean.
Electrophoresis Memo & Results: Write up a 2-page (typed, double spaced, 12 point, Times Roman font, 1” margins) discussion of your results, observations and conclusions. Include answers to the questions asked within this lab report. Please include Excel plots of your data and of the analysis. Please address the following question in the final paragraph; now that you are more familiar with electrophoresis, what else could this technology be used for?
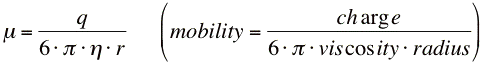
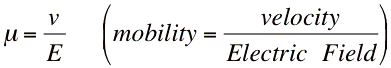
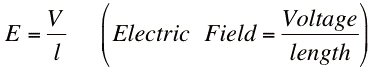
 EXPERIMENT
EXPERIMENT